Atoms are the fundamental systems of issue and also the specifying framework of components. The term “atom” originates from the Greek word for indivisible, since it was as soon as assumed that atoms were the tiniest points in deep space and also can not be separated. We currently recognize that atoms are comprised of 3 bits: electrons, neutrons and also protons– which are made up of also smaller sized fragments such as quarks.
Atoms were developed after the Big Bang 13.7 billion years back. As the warm, thick brand-new world cooled down, problems ended up being appropriate for electrons as well as quarks to develop. Quarks integrated to develop neutrons as well as protons, and also these bits integrated right into centers. This occurred within the initial couple of mins of deep space’s presence, inning accordance with CERN.
It took 380,000 years for deep space to cool off sufficient to reduce the electrons to ensure that the cores can record them to develop the very first atoms. The earliest atoms were mostly hydrogen as well as helium, which are still one of the most bountiful aspects in deep space.
Gravity at some point triggered clouds of gas to integrate and also create celebrities, and also larger atoms were (and also still are) developed within the celebrities and also sent out throughout deep space when the celebrity took off (supernova).
Daftar Isi
Atomic Structure
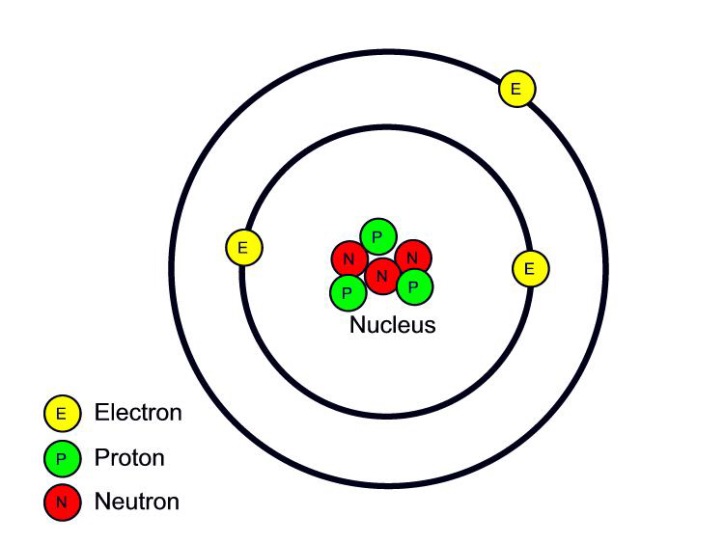
Protons as well as neutrons are much heavier compared to electrons and also live in the core at the facility of the atom. Electrons are incredibly light-weight as well as exist in a cloud orbiting the center. The electron cloud has a span 10,000 times above the core.
Protons as well as neutrons have around the very same mass. One proton evaluates even more compared to 1,800 electrons. Atoms constantly have an equivalent number of electrons as well as protons, as well as the number of protons and also neutrons is generally the very same. Including a proton to an atom makes a brand-new aspect, while including a neutron makes an isotope, or much heavier variation, of that atom.
Protons, Neutrons, and Electrons
Dalton’s Atomic Theory clarified a great deal regarding issue, chemicals, as well as chain reactions. It was not totally precise, since in contrast to just what Dalton thought, atoms can, in reality, be damaged apart right into smaller sized subunits or subatomic fragments.
We have actually been discussing the electron in terrific information, yet there are 2 various other bits of rate of interest to utilize: neutrons and also protons. We currently found out that J. J. Thomson uncovered an adversely billed bit, called the electron.
Rutherford suggested that these electrons orbit a favorable center. In succeeding experiments, he located that there is a smaller sized favorably billed bit in the center which is called a proton. There is a 3rd subatomic bit, referred to as a neutron.
Electrons
Electrons are among 3 major sorts of bits that compose atoms. The various other 2 kinds are neutrons as well as protons. Unlike neutrons and also protons, which contain smaller sized, less complex bits, electrons are essential fragments that do not include smaller sized bits.
They are a sort of essential bits called leptons. All leptons have an electrical cost of − 1 − 1 or 00. Electrons are exceptionally little. The mass of an electron is just around 1/2000 the mass of a proton or neutron, so electrons add practically absolutely nothing to the complete mass of an atom.
Electrons have an electrical cost of − 1 − 1, which is contrary yet equivalent to the cost of a proton, which is +1 +1. All atoms have the very same variety of electrons as protons, so the unfavorable as well as favorable fees “counteract”, making atoms electrically neutral.
Unlike neutrons and also protons, which lie inside the core at the facility of the atom, electrons are located outside the core. Adverse electrons are brought in to the favorable core due to the fact that contrary electrical costs draw in each various other. This pressure of tourist attraction maintains electrons regularly relocating with the or else void around the center.
The number listed below is an usual method to stand for the framework of an atom. It reveals the electron as a bit orbiting the core, just like the manner in which worlds orbit the sunlight. This is nevertheless, an inaccurate point of view, as electrons are extra made complex as quantum auto mechanics show.
Protons
A proton is among 3 major bits that comprise the atom. The various other 2 fragments are the neutron and also electron. Protons are discovered in the core of the atom. This is a small, thick area at the facility of the atom. Protons have a favorable electric fee of one (+1 )( +1) and also a mass of 1 atomic mass system (amu), which has to do with 1.67 × 10 −27 kilos. Along with neutrons, they comprise practically every one of the mass of an atom.
Neutrons
Atoms of all components – with the exception of a lot of atoms of hydrogen – have neutrons in their core. Unlike electrons and also protons, which are electrically billed, neutrons have on the house – they are electrically neutral. That’s why the neutrons in the layout over are identified n0n0. The no mean “absolutely no fee”.
The mass of a neutron is somewhat higher than the mass of a proton, which is 1 atomic mass device (amu)( amu). (An atomic mass device amounts to concerning 1.67 × 10 −27 kg.) A neutron likewise has concerning the very same size as a proton, or 1.7 × 1017 meters.
As you could have currently thought from its name, the neutron is neutral. To puts it simply, it has on the house whatsoever, as well as is as a result neither brought in to neither fended off from various other items. Neutrons remain in every atom (with one exemption), and also they’re bound along with various other neutrons as well as protons in the atomic center.
Prior to we carry on, we have to talk about exactly how the various sorts of subatomic bits connect with each various other. The response is apparent when it comes to neutrons. Because neutrons are neither brought in to neither driven away from items, they do not actually engage with electrons or protons (past being bound right into the center with the protons).
Although protons, neutrons, as well as electrons are all sorts of subatomic bits, they are not just the same dimension. When you contrast the masses of protons, electrons, and also neutrons, just what you discover is that electrons have an incredibly little mass, as compared to either neutrons or protons.
On the various other hand, the masses of neutrons as well as protons are rather comparable, although practically, the mass of a neutron is somewhat bigger compared to the mass of a proton. Since neutrons as well as protons are a lot extra huge compared to electrons, mostly all of the mass of any type of atom originates from the center, which consists of all the neutrons as well as protons.
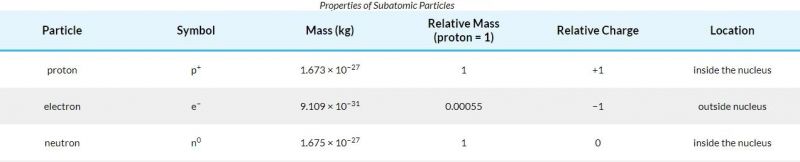
The table offers the homes as well as areas of protons, electrons, as well as neutrons. The 2nd column reveals the masses of the 3 subatomic fragments in “atomic mass devices.” An atomic mass device (amuamu) is specified as one-twelfth the mass of a carbon-12 atom. Atomic mass devices (amuamu) work, since, as you could see, the mass of a proton and also the mass of a neutron are virtually specifically 1.0 in this device system.
Positive as well as adverse costs of equivalent size terminate each various other out. This indicates that the unfavorable fee on an electron completely stabilizes the favorable cost on the proton. Simply puts, a neutral atom has to have specifically one electron for each proton.
It should have 1 electron if a neutral atom has 1 proton. It has to have 2 electrons if a neutral atom has 2 protons. It needs to have 10 electrons if a neutral atom has 10 protons. You understand. In order to be neutral, an atom needs to have the very same variety of protons and also electrons.
How You Can Find Number of Protons, Neutrons, and Electrons
Adhere to these basic actions to discover the variety of protons, neutrons, and also electrons for an atom of any type of aspect.
Fundamental Information About Elements
You’ll should collect fundamental info concerning the aspects to discover the variety of electrons, neutrons, and also protons. All you require is a regular table.
For any type of atom, exactly what you have to bear in mind is:
- Variety of Protons = Atomic Number of the Element
- Variety of Electrons = Number of Protons
- Variety of Neutrons = Mass Number – Atomic Number
Locate The Number of Protons
Each component is specified by the variety of protons located in each of its atoms. Regardless of the amount of electrons or neutrons an atom has, the component is specified by its variety of protons. The table of elements is organized in order of enhancing atomic number, so the variety of protons is the component number. For hydrogen, the variety of protons is 1. For zinc, the variety of protons is 30.
Locate The Number of Electrons
For a neutral atom, the variety of electrons coincides as the variety of protons.
Typically, the variety of electrons and also protons is not the exact same, so the atom lugs an internet favorable or unfavorable cost. If you understand its cost, you could identify the number of electrons in an ion. A cation lugs a favorable fee and also has even more protons compared to electrons An anion brings an unfavorable cost as well as has even more electrons compared to protons. Neutrons do not have a web electrical fee, so the variety of neutrons does not matter to the estimation.
The variety of protons of an atom could not transform, so you include or deduct electrons to obtain the right fee. If an ion has a 2+ fee, like Zn2+, this indicates there are 2 even more protons compared to electrons.
30 – 2 = 28 electrons.
If the ion has a 1- cost (just created with a minus superscript), after that there is even more electron compared to the variety of protons.
For F-, the variety of protons (from the table of elements) is 9 as well as the variety of electrons is:
9 + 1 = 10 electrons
Discover The Number of Neutrons
To discover the variety of neutrons in an atom, you have to discover the mass number for every component. The table of elements provides the atomic weight for each and every component, which could be made use of to discover mass number, For hydrogen, as an example, the atomic weight is 1.008.
Each atom has an integer variety of neutrons, yet the table of elements provides a decimal worth since it is a heavy standard of the variety of neutrons in the isotopes of each component. Exactly what you require to do is round the atomic weight to the local entire number to obtain a mass number for your computations. For hydrogen, 1.008 is closer to 1 compared to 2, so allow’s call it 1.
- Variety of Neutrons = Mass Number – Number of Protons = 1 – 1 = 0
- For zinc, the atomic weight is 65.39, so the mass number is closest to 65.
- Variety of Neutrons = 65 – 30 = 35
Experiments of Protons, Neutrons, and Electrons

Pupils could see proof of the fees of protons and also electrons by doing a task with fixed electrical power.
Keep in mind: When 2 products are massaged with each other in a fixed electrical energy task, one product has the tendency to shed electrons while the various other product has the tendency to acquire electron. In this task, human skin has the tendency to shed electrons while the plastic bag, made from polyethylene, has the tendency to acquire electrons.
Concern to explore
Exactly what makes things bring in or ward off each various other?
Products for each and every team
- Plastic grocery store bag
- Scissors
Procedure
Charged plastic and also charged skin
- Cut 2 strips from a plastic grocery store bag to ensure that each has to do with 2– 4 centimeters broad as well as concerning 20 centimeters long.
- Hold the plastic strip strongly at one end. Realize the plastic strip in between the thumb as well as fingers of your various other hand as revealed.
- A pupil holds a lengthy strip of plastic in between her thumb as well as index figers.
- Promptly draw your leading hand up to make sure that the plastic strip goes through your fingers. Do this 3 or 4 times.
- Permit the strip to suspend. Bring your various other hand near it.
- Compose “bring in” or “drive away” in the graph on the task sheet to explain exactly what occurred.
Expected outcomes
The plastic will certainly be drawn in to your hand and also approach it. Pupils might see that the plastic is likewise drawn in to their sleeves and also arms. Allow pupils understand that later on in this lesson they will certainly explore why the plastic strip is likewise drawn in to surface areas that have actually not been billed (neutral).
Keep in mind: If trainees locate that their plastic strip does stagnate towards their hand, it has to not have actually been billed all right. Have them attempt billing their plastic strip by quieting on their trousers or t shirt and afterwards rapidly drawing it with the various other hand. They must evaluate to see if the plastic is brought in to their garments. Otherwise, pupils must attempt billing the plastic once again.
Summary

Atoms, protons, electrons and also neutrons are the standard foundation of issue. Protons as well as neutrons comprise the center of an atom, while electrons circle this core. The variety of these bits that comprise an atom are exactly what aid distinguish aspects from each other, with aspects consisting of even more protons provided greater on the routine graph.
Atom
An atom is consisted of a core consisting of protons and also neutrons, in addition to electrons that orbit the center. This pointlike bit is the smallest item that could maintain the homes of a component. It could not be separated or separated by any kind of chemical approaches.
Electron
An electron is bound to and also orbits the core of an atom. This indivisible fragment has an adverse fee, typically described as “minus 1.” Its mass is 1/1,837 of the mass of a proton.
Neutron
Situated in the core of atoms, neutrons have mass somewhat less than those of protons. This indivisible fragment obtains its name for that it has no electric cost. It is 1,839 times the dimension in mass of an electron.
Proton
Aspects obtain their atomic number based upon the variety of protons discovered in each atom. This indivisible bit in the core of an atom brings a favorable fee, described as “1” on the atomic weight range. A proton has a mass 1,837 times higher than that of an electron.
Atoms constantly have an equivalent number of electrons as well as protons, and also the number of protons and also neutrons is generally the very same. The mass of an electron is just around 1/2000 the mass of a proton or neutron, so electrons add practically absolutely nothing to the complete mass of an atom.
Because neutrons are neither drawn in to neither warded off from items, they do not actually engage with electrons or protons (past being bound right into the core with the protons).
When you contrast the masses of neutrons, protons, and electrons, exactly what you locate is that electrons have an exceptionally tiny mass, contrasted to either neutrons or protons. Since neutrons as well as protons are so a lot extra substantial compared to electrons, practically all of the mass of any type of atom comes from the core, which includes all of the neutrons and protons.